Common Issues & Solutions For Ophthalmic Cannula Forming Operations
In the ophthalmic cannula forming industry, pain points persist in the areas of ergonomic and operator safety, consistency, quality, profitability,...
2 min read
 John A. Weismantel
:
Aug 1, 2024 8:30:00 AM
John A. Weismantel
:
Aug 1, 2024 8:30:00 AM

In part one of this blog series, here we covered common obstacles with humans vs. automated medical device assemblies. We also covered issues with variations in raw materials for cannula forming and then finally quality process control on the production line. Ophthalmic cannula forming operations benefit greatly from automation if you select the right automation partner to address top processing challenges on the line. We will pick back up with common problems in manual cannula-forming operations with changeover procedures.
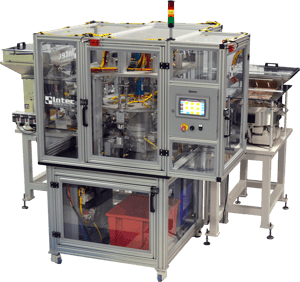 A changeover procedure for forming tools can be a huge pain point in cannula manufacturing.
A changeover procedure for forming tools can be a huge pain point in cannula manufacturing.
Companies that run a high mix, low volume production schedule benefit greatly from quick tooling changeovers, especially in plants that changeover multiple times a day. Tooling changeovers on Intec equipment are fast and do not introduce quality risks.
Forming tools produce a very specific geometry, as well as the radius and the angles of needles. These forming tools are usually composed of a male and female forming tool, and during manufacturing, a needle gets bent across an opposite forming tool in a station.
Intec uses SME (Single Minute Exchange) methodology when designing the way forms are exchanged. This methodology makes it very easy to take the old forms out and put the new forms in, and they self-align so that there is no chance that a human can position them incorrectly. Angle bends can be automated to a further degree with the use of servo-driven forming tools. Specific angle values & the starting position of the bend can be recipe-driven and adjusted through the HMI. This allows one toolset to produce many variations of finished products without the need for a change-over
The time savings are significant, from tens of minutes down to less than a minute, with the total amount of time savings dependent on how often product numbers change during a production schedule.
Another pain point in cannula manufacturing is downstream packaging. Because cannulas are single-use components, they are normally pouched individually in a package that peels apart.
Most cannula manufacturers today re-sheathed their formed cannulas into a bulk bin. The customer must then take them to a downstream processor for final packaging.
If you're tired of that routine or want to bring the downstream processing task in-house and into your control, Intec has experience pouching machines for use immediately after forming.
If you think about it, the cost of quality is very market-specific, and if you are manufacturing medical devices that are used in critical, life-altering surgeries, then quality is of the utmost importance.
That's why eliminating your pain points will increase the consistency, quality, profitability, and efficiency of your cannula manufacturing line.
In the ophthalmic cannula forming industry, pain points persist in the areas of ergonomic and operator safety, consistency, quality, profitability,...
Automation is adding new efficiencies across every manufacturing sector due to a constrained labor market. For medical manufacturers, the growing...
Recent advances in a variety of prophylactic and curative treatments have increased the demand for application specific custom bent or formed needles...