INTEC OFFERS TURNKEY MACHINE BUILDING
Intec Automation offers turnkey services for its machine building capabilities from collaborating with customers on the best concept of the project...
2 min read
 John A. Weismantel
:
Oct 16, 2025 11:59:59 AM
John A. Weismantel
:
Oct 16, 2025 11:59:59 AM

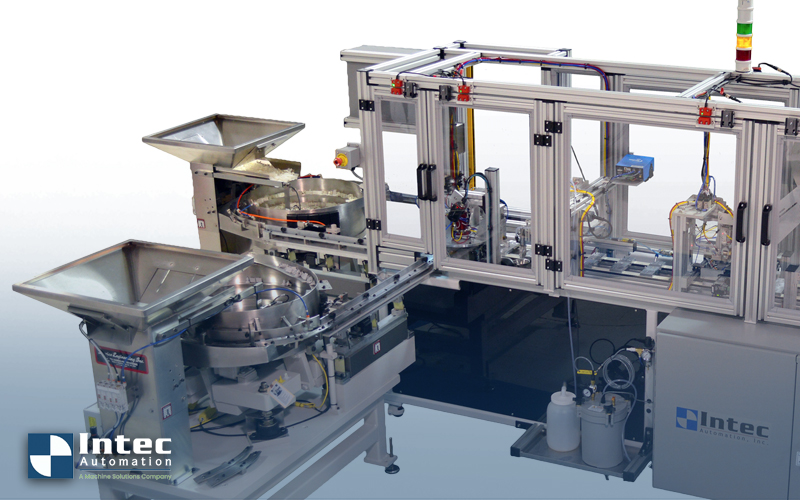
Medical device manufacturers face a unique challenge when producing consumables such as diagnostic cartridges, IV catheters, and surgical components. These assemblies often fall into the “low mix, high volume” category or limited product variation but massive daily output. Meeting that demand with manual processes stretches labor, risks quality, and drives up costs, especially under strict regulatory oversight. Automation provides the consistency and throughput these assemblies require.
 Low mix, high volume manufacturing depends on repetitive accuracy. In a manual line, speed and balance are dictated by the slowest operator. Staffing multiple shifts doesn’t help the problem either, as line productivity often drops in later shifts. High turnover drives up costs, and every new hire requires more retraining. Not to mention the difficulty in hiring qualified workers in today’s environment. That constant cycle also creates inconsistency on the line. Small mistakes (too much adhesive in one spot or a slight misalignment) can mean scrap or even a recall. And even skilled operators have off days, where fatigue or distraction makes manual handling less dependable than automation.
Low mix, high volume manufacturing depends on repetitive accuracy. In a manual line, speed and balance are dictated by the slowest operator. Staffing multiple shifts doesn’t help the problem either, as line productivity often drops in later shifts. High turnover drives up costs, and every new hire requires more retraining. Not to mention the difficulty in hiring qualified workers in today’s environment. That constant cycle also creates inconsistency on the line. Small mistakes (too much adhesive in one spot or a slight misalignment) can mean scrap or even a recall. And even skilled operators have off days, where fatigue or distraction makes manual handling less dependable than automation.
Unlike high mix, low volume operations, which require rapid changeovers (flexible feeders, for example), low mix, high volume lines are ideal for dedicated automation. Once the equipment is dialed in, every cycle repeats with precision. Automation not only stabilizes output but also frees engineering teams from constant oversight of operator performance. In regulated environments, that consistency translates directly to reduced compliance risk.
 The foundation of automation is selecting equipment that matches the product and production goals:
The foundation of automation is selecting equipment that matches the product and production goals:
Together, these layers create a repeatable workflow where every part is built and verified to specification.
Most automated equipment isn’t built piece by piece on the production floor. It’s engineered and tested as a complete line in advance. After passing factory acceptance testing, the system is shipped in and set up to run as a self-contained unit. This way, production teams don’t lose weeks trying to stitch automation into a manual process. Instead, they bring the line online quickly and start seeing output improve almost immediately.
For medical assemblies, speed alone is not enough. Automation must maintain cleanliness, accuracy, and traceability to satisfy regulatory standards. Every inspection camera, sensor, and test station contributes to a closed-loop system that documents compliance while maximizing throughput. The result is a line that can run continuously with fewer operators, lower training costs, and a more consistent product output.
Contact Intec Automation today to discuss how purpose-built equipment can streamline your low mix, high volume medical assemblies.

Intec Automation offers turnkey services for its machine building capabilities from collaborating with customers on the best concept of the project...
Over the last 30 years, the labor participation rate in the U.S. experienced a steady decline. What analysts are now referring to as the “forever”...

Dispensing machinery is playing an increasingly important role in medical device assembly in the healthcare and life sciences industries. While...