Medical Device OEMs: How to Ensure Zero Out-of-Box Failures
Product failures borne from low-quality design or inadequate manufacturing processes are simply unacceptable for medical device OEMs. The liabilities...
1 min read
 Juan Cardenas
:
Apr 18, 2024 10:00:00 AM
Juan Cardenas
:
Apr 18, 2024 10:00:00 AM

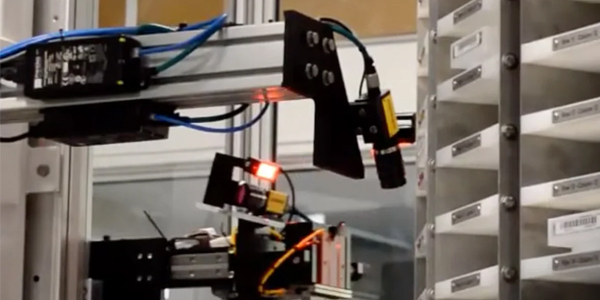
Out-of-box failures can stem from a myriad of factors. For bent needles or cannulas, the geometry of the final product has to conform to the exact dimensions required for its intended use. Similarly, process parameters can be sensitive to variations in temperature and humidity (especially when it comes to filling or dispensing exact volumes). Poorly maintained machines, environmental factors, and human operators can contribute to out-of-box failures.
Controlling each of these variables across batches requires extensive quality control measures that detect and intervene when products pass through different assembly stations.
These solutions can integrate with a robust machine design that addresses each of the quality checks required during manufacturing. Automated reject functions can remove defective raw materials or products as they fail to eliminate added additional downstream value and only allow compliant devices to leave the line.
Product failures borne from low-quality design or inadequate manufacturing processes are simply unacceptable for medical device OEMs. The liabilities...
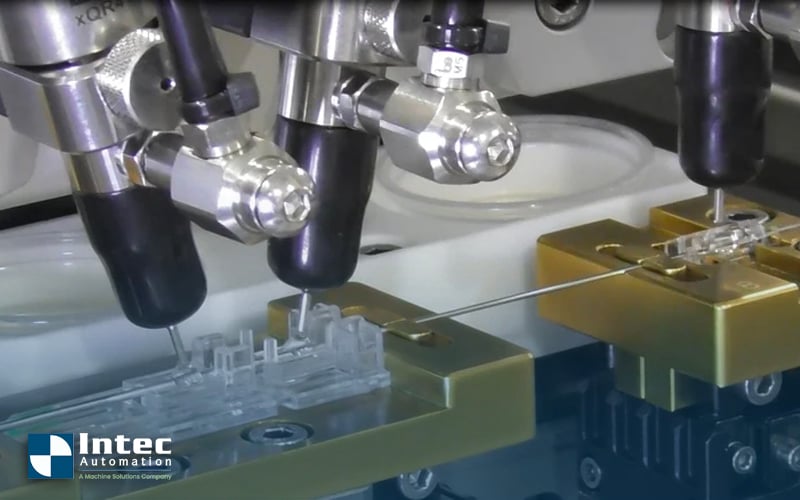
Achieving exact tolerances in micro-dispensing liquid applications is critical for ensuring precision, consistency, and functionality in high-tech...

Manual steps remain common in diagnostic device assembly, but they often create more challenges than benefits. High output requirements and strict...