Automated Resistance and HiPot Testing to Detect Conductive Failures in Circuits
With more sophisticated electronics powering our daily lives, manufacturers that want to automate their assembly operations have to incorporate...
3 min read
 John A. Weismantel
:
Jun 26, 2023 11:00:00 AM
John A. Weismantel
:
Jun 26, 2023 11:00:00 AM
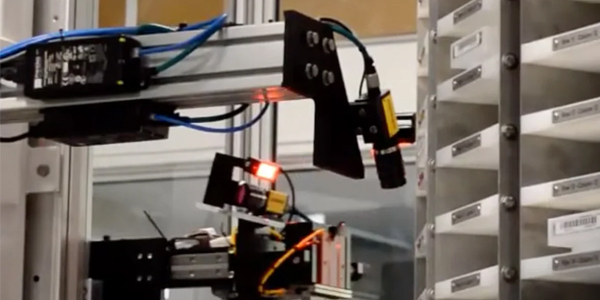
Product failures borne from low-quality design or inadequate manufacturing processes are simply unacceptable for medical device OEMs. The liabilities that can arise from malfunctioning machines, damaged consumables, or faulty surgical instruments once it arrives at the end-user may leave your brand DOA.
 Making the case for zero out-of-box failures in medical manufacturing environments shouldn’t be a debate. When manufacturing medical devices, including sutures, needles, catheters, or any other surgically invasive products, you have to maintain the strict quality standards set out by the FDA and WHO. A few years ago, McKinsey analyzed the costs of poor quality for medical device OEMs and found:
Making the case for zero out-of-box failures in medical manufacturing environments shouldn’t be a debate. When manufacturing medical devices, including sutures, needles, catheters, or any other surgically invasive products, you have to maintain the strict quality standards set out by the FDA and WHO. A few years ago, McKinsey analyzed the costs of poor quality for medical device OEMs and found:
We should note that McKinsey conducted this research before the global health crisis, which increased the demand for diagnostic and therapeutic devices exponentially. In the same vein, medical OEMs can maximize their revenues using manufacturing technologies that ensure zero out-of-box failures with investments in process automation.
 Out-of-box failures can stem from a myriad of factors. For bent needles or cannulas, the geometry of the final product has to conform to the exact dimensions required for its intended use. Similarly, process parameters can be sensitive to variations in temperature and humidity (especially when it comes to filling or dispensing exact volumes). Manufacturers also have to confirm that any raw materials meet the product specifications before spending time and effort to produce an item that won’t pass the final validation checks. Badly maintained machines, environmental factors, and human operators can also contribute to out-of-box failures.
Out-of-box failures can stem from a myriad of factors. For bent needles or cannulas, the geometry of the final product has to conform to the exact dimensions required for its intended use. Similarly, process parameters can be sensitive to variations in temperature and humidity (especially when it comes to filling or dispensing exact volumes). Manufacturers also have to confirm that any raw materials meet the product specifications before spending time and effort to produce an item that won’t pass the final validation checks. Badly maintained machines, environmental factors, and human operators can also contribute to out-of-box failures.
Controlling each of these variables across batches requires extensive quality control measures that detect and/or intervene when products pass through different assembly stations.
Relying on manual operators and benchtop equipment to perform these processes consistently and repeatedly increases cycle times and may lead to poor-quality products coming off the line. Depending on the application and design tolerances, maintaining part-to-part repeatability is a key concern for medical manufacturers. Failures can lead to reputational damage, legal liability, and loss in profits when there aren’t adequate quality checks built into the process.
Integrating inline inspections into an automated manufacturing machine cell can alleviate these risks by consistently performing the same tasks repeatedly, without ever allowing any defective product to leave the line.
Process automation enables manufacturers to control every variable in the production process. Vision systems can accommodate differences in raw materials and adjust the process parameters to ensure the product meets the required specifications. Additional validation checks can ensure that the system dispensed the right volume of liquid into a tube and verify that the required agitation agent (such as a ball bearing) is present in the medical assemblies.
 Examples of automated quality validation checks can include:
Examples of automated quality validation checks can include:
These solutions can integrate with a robust machine design that addresses each of the quality checks required during manufacturing. Automated reject functions can remove defective raw materials or products as they fail any check and only allow compliant devices to leave the line. Intec Automation provides integrated manufacturing machine cells that provide inline quality validation and inspection systems to ensure zero out-of-box failures in complex medical assemblies.
Automation is the most cost-effective way to ensure repeatability and reduce product liability concerns. It allows manufacturers to increase productivity and maintain the required quality of each item by inspecting, validating, and rejecting nonconformant items automatically. As medical device OEMs have to adhere to strict internal standards and external regulations, establishing an automated quality validation process during manufacturing operations is the best way to eliminate out-of-box failures in the future.
To discuss your requirements with the automation experts, start a conversation with Intec Automation to see how we can help you achieve zero out-of-box failures.
With more sophisticated electronics powering our daily lives, manufacturers that want to automate their assembly operations have to incorporate...

Cannulas are small tubes that are primarily used to deliver or extract fluid. They are used in many different industries, with a high percentage...

After a few years of turmoil, analysts now have a positive outlook on the US manufacturing sector. Supply chains are stabilizing and new production...