Needle-Bending Equipment Solutions for Medical Manufacturers to Minimize Downtime
Recent advances in a variety of prophylactic and curative treatments have increased the demand for application specific custom bent or formed needles...
2 min read
 Matthew Bresnahan
:
May 10, 2023 9:00:00 AM
Matthew Bresnahan
:
May 10, 2023 9:00:00 AM
Nearly half – 44% – of manufacturers have experienced year-over-year cost increases in medical device assembly technology in the last two years. With significant supply chain uncertainties and other economic disruptions continuing in 2023, many manufacturers are focused on ways to reduce unnecessary costs.
In complex product assembly, shifting from manual operations to automated assembly systems, either entirely or in part, provides one of the shortest and most reliable paths to reduce quality issues and allow for labor reallocation to high value areas.
 In any industry, “complex” is a relative term. Nevertheless, medical device assembly often involves tasks that require a high degree of dexterity and constant visual feedback on the part of human operators. Such complex tasks include bending materials and products like needles to specified orientations or manipulating webbed materials on multiple axes to install them on other device components.
In any industry, “complex” is a relative term. Nevertheless, medical device assembly often involves tasks that require a high degree of dexterity and constant visual feedback on the part of human operators. Such complex tasks include bending materials and products like needles to specified orientations or manipulating webbed materials on multiple axes to install them on other device components.
While some complex assembly tasks remain unsuitable for full automation, applicable product URSs don’t always break specified assembly steps down thoroughly, leaving many simple, intermediary tasks better suited for automation in the hands of human operators. As automation can increase both throughput and reliability for simpler subtasks, device manufacturers should evaluate assembly processes granularly to identify complex tasks within as narrow a window as possible and reveal manual operations suitable for automation.
 As medical devices remain subject to FDA validations as long as they’re in use, the ability to monitor performance parameters during assembly is critical to completing necessary inspections and preventing costly disruptions during process audits. Tracking parameters such as tension, PSI, or temperature allows manufacturers to maintain comprehensive production records for devices and quickly identify root causes when errors occur.
As medical devices remain subject to FDA validations as long as they’re in use, the ability to monitor performance parameters during assembly is critical to completing necessary inspections and preventing costly disruptions during process audits. Tracking parameters such as tension, PSI, or temperature allows manufacturers to maintain comprehensive production records for devices and quickly identify root causes when errors occur.
Inspection processes that automated solutions can efficiently monitor for specific parameters recorded with database tracking include bonding, pressing assemblies together, electrical, and functional testing, gluing, and UV curing.
 Designing efficient assembly processes that maximize opportunities to reduce unnecessary manual operations requires a thorough audit of process steps and articulating how an operator puts something together. Without an understanding of unique operator tasks that enable subsequent assembly steps, automation plans can easily bottleneck, nullifying their intended advantages.
Designing efficient assembly processes that maximize opportunities to reduce unnecessary manual operations requires a thorough audit of process steps and articulating how an operator puts something together. Without an understanding of unique operator tasks that enable subsequent assembly steps, automation plans can easily bottleneck, nullifying their intended advantages.
Incomplete auditing of process steps – often not explicitly outlined in product URSs – commonly result in manufacturers overlooking opportunities to improve quality and reduce product variability through automation. Similarly, manufacturers should assess automation possibilities according to the material and geometric features of assembly processes. For example, if steps involve too high a degree of multi-axis manipulation in a confined space or constant visual inspection of soft materials, full automation plans may not be viable.
At Intec Automation, we excel at analyzing complex medical device assembly operations and identifying areas where automation will improve efficiency and reliability with timely ROI. We tailor our solutions to the unique needs of your operation, evaluating processes for ergonomic and scaling concerns so that manufacturers enjoy sustained ROI over the lifetime of the equipment. With over 20 years of experience helping many of the industry’s largest medical device manufacturers, Intec offers clients not just automation services and solutions, but rather ongoing partnerships with a proven track record of long-term customer satisfaction.
To learn more about Intec custom automation solutions, contact us today.
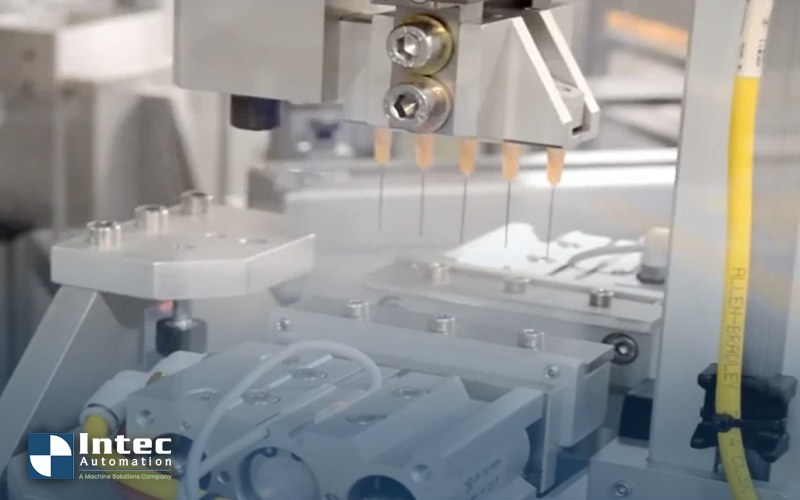
Recent advances in a variety of prophylactic and curative treatments have increased the demand for application specific custom bent or formed needles...

With its deep-rooted foundation of over two decades of experience in factory automation, specialty equipment and clean-sheet design and build of...

Matt Bresnahan, Intec’s General Manager, explains the six project stages involved in creating a custom automation solution. He used the example of...