It's critical to ask the right questions when searching for a partner to help you automate manufacturing processes in the medical and life sciences industry. Questions around issues such as quality and defects, time to market, speed, and experience, must be asked before the right partner is chosen. Our eBook will give you more guidance, but here are a few of the considerations to help you get started.
Will automation increase quality and reduce defects?
Quality and defects are concerns that many manufacturers face today. In a space where there are a lot of operators, there are often quality issues because of manual operations or assembly environmental factors such as debris. The most notable way to control quality issues is by reducing manual operations, so there are no deviations to the process. An automated system results in the same consistency every time while reducing the risk of contamination for these sensitive operations.
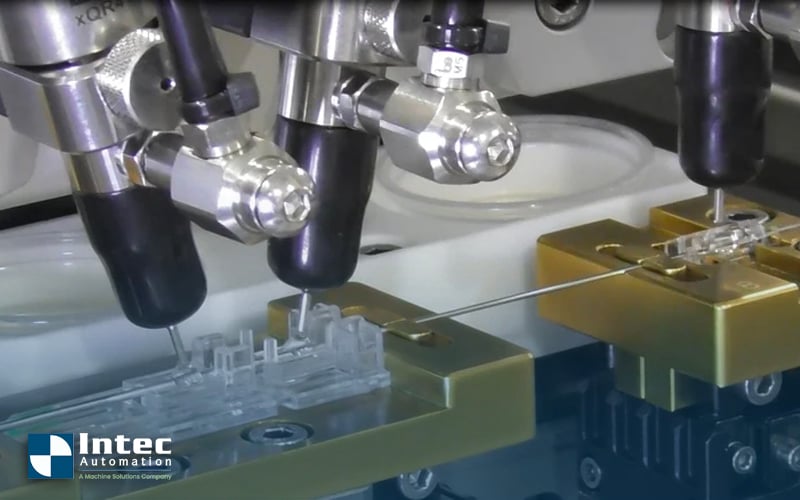
 Intec Automation recently helped a customer develop an automated manufacturing process that solved their quality and defect concerns. This customer needed to weld very fine materials onto a plastic tray where cancer cultures are grown. The materials were so sensitive that even touching the surface would destroy the product. Automating the task meant that the human touch was eliminated in the welding process while monitoring and tracking each step to ensure the quality of each product.
Intec Automation recently helped a customer develop an automated manufacturing process that solved their quality and defect concerns. This customer needed to weld very fine materials onto a plastic tray where cancer cultures are grown. The materials were so sensitive that even touching the surface would destroy the product. Automating the task meant that the human touch was eliminated in the welding process while monitoring and tracking each step to ensure the quality of each product.
Intec also recently partnered with other customers to help develop medical application automation equipment for pharmaceutical-grade filter materials applications, culture cell growth assemblies, PCR tubes for molecular biology applications, and multi-wells for laboratory applications. Many of the common problems come back to the customer looking for:
- increased precision and accuracy for consistent, high-volume output at the lowest cost possible
- improved uptime and productivity
- reducing the risk of contamination
- producing products in varying operating conditions such as lower temperatures, humidity, and light which is possible to dictate with machines but manual constraints require specific conditions for safety and comfort requirements for operations
 The medical and life sciences sector has several unique requirements and standards for automation equipment that are not found in other industries. Many times, medical applications will require cleanroom standards, so the materials and construction of equipment are important because of the higher level sanitation regime. Equipment must be easy to washdown and use materials and practices that reduce harborage points where contaminants can exist.
The medical and life sciences sector has several unique requirements and standards for automation equipment that are not found in other industries. Many times, medical applications will require cleanroom standards, so the materials and construction of equipment are important because of the higher level sanitation regime. Equipment must be easy to washdown and use materials and practices that reduce harborage points where contaminants can exist.
Intec Automation helps its customers get through the maze of validation and standards requirements in the life science industry, including design qualifications that are not required outside of the medical space. Other validation services are made available through Intec's sister company.
What is a typical lead time for an automation project?
Time to market is another critical consideration when you are working with assembly automation companies. Validating products can be a lengthy process, and a machine can sit on a customer's floor for a full year before it is fully validated. That's why it is important that the partner you choose communicates honestly about expectations and lead time.
Intec understands that the faster they can deliver a system to a customer, the faster that customer can begin their validation process and improve their time to market.
Will an automated system really give me the speed that I need?
 Speed is driving automation in manufacturing today, and manual operations can be fraught with speed bumps. Removing those speed bumps with automation and increasing throughput will reduce defects and waste percentages.
Speed is driving automation in manufacturing today, and manual operations can be fraught with speed bumps. Removing those speed bumps with automation and increasing throughput will reduce defects and waste percentages.
Companies report that their OEE (Overall Equipment Effectiveness) is in the 50-55% range for manual operations. However, when they go to full automation, that OEE number jumps up to around 85%, which is a huge improvement in just one stage of the operation.
Does your company have experience building solutions for my type of products?
Precision and accuracy are critical in the life science industry, so when you begin your search for a partner, you must find someone experienced in creating high-tolerance precision solutions for the types of products that you have.
Intec Automation has over 20 years of experience successfully designing and building custom solutions for manufacturers all over the world, and we can get you on the path to successfully automating your manual processes.
For more questions to ask, check out our eBook, which is a guide to selecting a partner for future automated manufacturing solutions for your production lines.
If you want to secure your profitability with an automated manufacturing solution, speak to one of our engineers today.



 Dane Mooers
Dane Mooers

 Intec Automation recently helped a customer develop an automated manufacturing process that solved their quality and defect concerns. This customer needed to weld very fine materials onto a plastic tray where cancer cultures are grown. The materials were so sensitive that even touching the surface would destroy the product. Automating the task meant that the human touch was eliminated in the welding process while monitoring and tracking each step to ensure the quality of each product.
Intec Automation recently helped a customer develop an automated manufacturing process that solved their quality and defect concerns. This customer needed to weld very fine materials onto a plastic tray where cancer cultures are grown. The materials were so sensitive that even touching the surface would destroy the product. Automating the task meant that the human touch was eliminated in the welding process while monitoring and tracking each step to ensure the quality of each product. The medical and life sciences sector has several unique requirements and standards for automation equipment that are not found in other industries. Many times, medical applications will require cleanroom standards, so the materials and construction of equipment are important because of the higher level sanitation regime. Equipment must be easy to washdown and use materials and practices that reduce harborage points where contaminants can exist.
The medical and life sciences sector has several unique requirements and standards for automation equipment that are not found in other industries. Many times, medical applications will require cleanroom standards, so the materials and construction of equipment are important because of the higher level sanitation regime. Equipment must be easy to washdown and use materials and practices that reduce harborage points where contaminants can exist. Speed is driving automation in manufacturing today, and manual operations can be fraught with speed bumps. Removing those speed bumps with automation and increasing throughput will reduce defects and waste percentages.
Speed is driving automation in manufacturing today, and manual operations can be fraught with speed bumps. Removing those speed bumps with automation and increasing throughput will reduce defects and waste percentages.