Common Problems & Solutions For Ophthalmic Cannula Forming Operations (Part 2)
In part one of this blog series, here we covered common obstacles with humans vs. automated medical device assemblies. We also covered issues with...
2 min read
 Juan Cardenas
:
Jun 28, 2023 10:30:00 AM
Juan Cardenas
:
Jun 28, 2023 10:30:00 AM

For manufacturers evaluating new projects in heavily regulated fields, such as medical device assembly, knowing how to calculate ROI for automation is critical for ensuring profitability on fixed budgets. Most manufacturers can accurately assess hard costs associated with running a set number of human operator shifts in an existing facility. However, adequately accounting for the accumulation of soft costs and liabilities introduced by human operators can be tough. Semi-to-full automation in assembly processes can reduce or eliminate many of these soft costs while also reducing hard costs.
The spread of soft costs in regulated assembly processes for products such as medical devices derive primarily from the ergonomic inefficiencies associated with accommodating human operators in production spaces, and the impact of variable output on validation processes.
 Assembly operations involving human workers must prioritize worker safety and provide appropriate working conditions. Compared to fully automated assembly systems, manual or semi-automated processes accrue additional costs in utility expenses such as lighting, climate control, and air filtration – which facilities must run any time workers are present.
Assembly operations involving human workers must prioritize worker safety and provide appropriate working conditions. Compared to fully automated assembly systems, manual or semi-automated processes accrue additional costs in utility expenses such as lighting, climate control, and air filtration – which facilities must run any time workers are present.
Manual processes also introduce long-term risks that escalate over time. Repetitive manual assembly tasks performed by the same operators for years commonly result in employee insurance claims and injury compensation. Repetitive motion injuries currently rank among the top injuries named in employee compensation awards in lawsuits filed against employers. The consequences of employee injury lawsuits are not limited to personal financial compensation but may also include expensive court-mandated changes to workplace layouts to reduce repetitive motion tasks and prevent future injuries.
In regulated manufacturing operations such as medical device assembly, manufacturers must satisfy two FDA standards for device production: verification and validation.
For the FDA’s purposes, verification means the design stage process confirms that a device meets specified product requirements. Validation is the process of proving that a device meets end user needs in practice, while conforming to applicable safety regulations.
As verification applies to design, it is a one-time process for medical device manufacturers. However, validation standards always apply to products in use. As such, any output variability or lack of consistency affects – and potentially jeopardizes – existing product validations. When medical device failures occur, the FDA requires product traceability to evaluate assembly processes and prevent subsequent failures.
 For manufacturers, the inherent inconsistencies of manual assembly operations represent persistent risks to patients, healthcare professionals, and client relationships. Compared to fully automated assembly modes – which result in lower product rejection rates - the variability associated with manually completed tasks will always increase the likelihood of downstream consequences such as product or lot recalls and mandated process testing.
For manufacturers, the inherent inconsistencies of manual assembly operations represent persistent risks to patients, healthcare professionals, and client relationships. Compared to fully automated assembly modes – which result in lower product rejection rates - the variability associated with manually completed tasks will always increase the likelihood of downstream consequences such as product or lot recalls and mandated process testing.
Across the board, assembly costs in the U.S. are on the rise. Under these conditions, medical device manufacturers cannot afford to ignore the soft costs and risks tied to manual assembly processes. At Intec Automation, we specialize in creating tailored automation solutions for fixed budgets. Intec solutions support long-term growth and change with options ranging from temporary human operator cells for approval processes to full assembly automation.
To learn more and discuss options with our sales team, contact us today.

In part one of this blog series, here we covered common obstacles with humans vs. automated medical device assemblies. We also covered issues with...

Over the last few decades, contract manufacturing has become an effective business strategy for expanding organizations. However, recent disruptions...
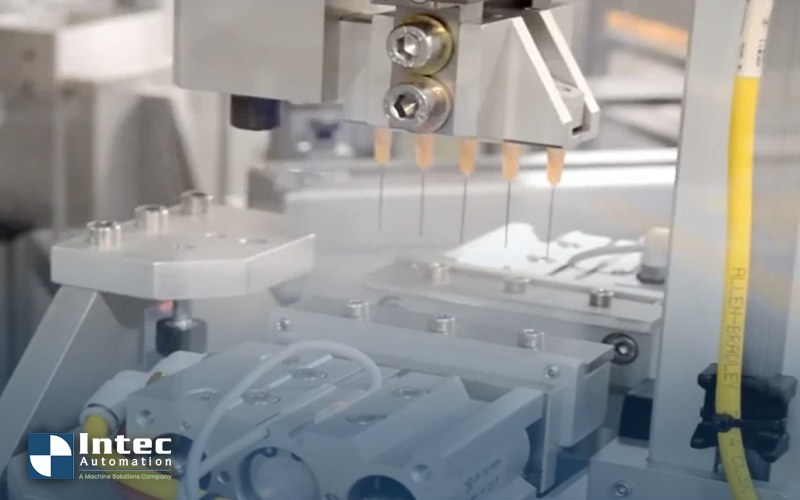
Recent advances in a variety of prophylactic and curative treatments have increased the demand for application specific custom bent or formed needles...