Applications of Automation in Medical Kit Assembly
In the medical industry, many of the tools used by medical professionals for patient diagnosis and treatment procedures are single-use. Examples...
1 min read
 John A. Weismantel
:
Sep 18, 2024 9:00:00 AM
John A. Weismantel
:
Sep 18, 2024 9:00:00 AM

When it comes to maintenance, we recommend all manufacturers develop a consistent schedule of routine and semiannual maintenance to prolong equipment’s working life, prevent downtime, maintain quality, and protect employees.
Even if you already incorporate routine and scheduled maintenance into your facility’s operations, you may be missing out on the full benefits if you don’t have a set of clear written procedures. Without thorough documentation, employees may be unclear on the latest protocols, and it is difficult to ensure consistent work. Maintenance procedures should outline, in detail and in writing, how personnel should inspect, clean, and repair equipment.
It should also delineate safe operating limits so that there is never doubt as to whether a situation calls for repair or replacement. In addition to documenting maintenance protocols, you should require written maintenance logs so that you can track equipment status and maintenance compliance over time.
And, the smartest manufacturers know, that although maintenance requires an upfront investment, it offers many key benefits, including minimizing downtime, improved safety, increased efficiency, and cost savings.
As always, Intec has you covered. Our data-driven, outcome-based industrial maintenance services provide specific support to help you maximize productivity, minimize operational risk, and meet your business goals.
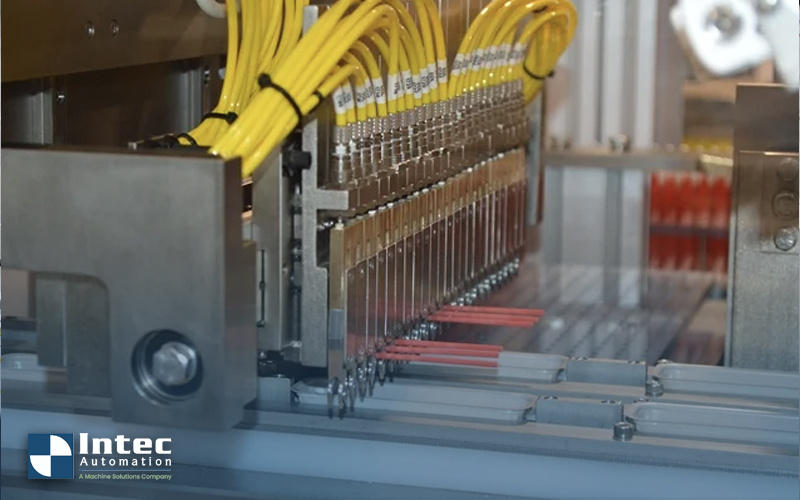
In the medical industry, many of the tools used by medical professionals for patient diagnosis and treatment procedures are single-use. Examples...
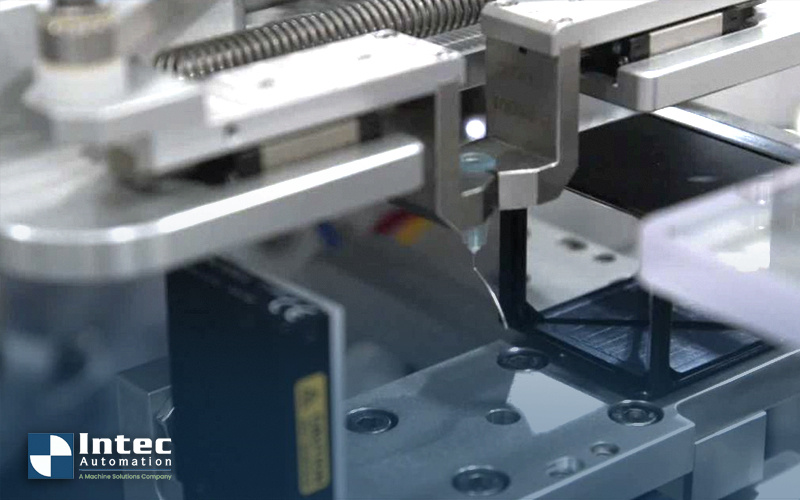
John, our VP of Engineering at Intec Automation, is diving deep into the different considerations for needle bending equipment, tooling, and...
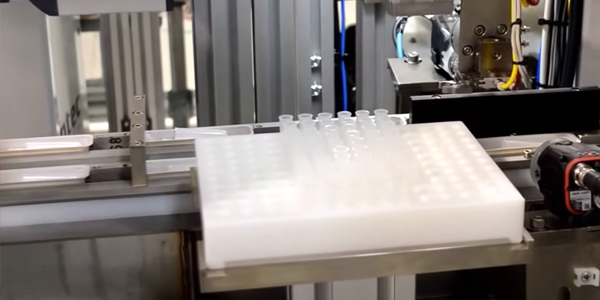
Automation has increasingly become a necessity for manufacturers of medical kits and medical device equipment to stay competitive and to keep up with...